Question bank
Chapter-1 Kinetic Molecular Theory of Matter
Q-1
Define the following terms and answer the associated questions:
(i) Matter : Describe a simple experiment to demonstrate that matter occupies space.
(ii) Deposition: Which of the following do not sublime at all - snow, camphor, dry ice, mercury, iodine?
(iii) Gas: State the condition necessary for gases to diffuse.
(iv) Solid: State the characteristic of solids that makes them rigid.
(v) Liquid: How many free surfaces do liquids have?
Q-2
Name the phenomenon that causes the following occurrences:
(i) Cloud formation
(ii) Water cycle in nature
(iii) Naphthalene balls become smaller in size on prolonged exposure to air.
(iv) Powdered naphthalene turns to liquid at 80°C.
(v) Formation of frost on leaves and grass.
(vi) Appearance of morning dew.
(vii) Water spilled on the floor disappears after a while.
(viii) Water droplets appear on the outer surface of a bottle taken out of the fridge and kept at room temperature.
Q-3
Distinguish between the following pairs on the basis of the kinetic molecular theory of matter.
(i) Sublimation and Deposition
(ii) Boiling and Evaporation
(iii) Freezing and Melting
Q-4
State whether the following statements are true or false:
(i) Interconversion of matter does not involve change in the mass of the substance.
(ii) Sublimation can occur at any temperature.
(iii) An increase in pressure lowers the melting point of a solid .
(iv) Intermolecular attraction is maximum in solids.
(v) Gases have innumerable free surfaces.
Q-5
Fill in the blanks:
(i) The irregular motion of suspended particles in a liquid is called _______ motion.
(ii) When a solid is heated, its internal kinetic energy ________
(iii) _______ is a rapid process and takes place at a fixed temperature.
(iv) The heat needed to change a solid into a liquid at the same temperature is called latent heat of _________.
(v) If the temperature _______ , Brownian motion also _________
Multiple Choice Questions
Q-1 The state in which the molecules can move freely within the boundary of the substance is:
(i)
Solid
(ii)
Liquid
(iii)
Gas
(iv)
Plasma
Q-2 Carbon dioxide can be liquefied by:
(i)
All of the above
(ii)
Increasing the temperature
(iii)
Compressing the gas and suddenly releasing the pressure
(iv)
Decreasing the pressure of the gas
Q-3 The Brownian motion increases with the:
(i)
Increase in density of the medium
(ii)
Decrease in temperature
(iii)
Decrease in the density of the medium
(iv)
Decrease in size of particles
Q-4 Which statement is True for the molecular structure of solids?
(i)
Atoms vibrate about their mean position
(ii)
Intermolecular space is negligible
(iii)
lntramolecular attraction is strong
(iv)
All statements are true
Q-5 The change from the solid state to liquid state on heating at a fixed temperature is called:
(i)
Melting
(ii)
Sublimation
(iii)
Freezing
(iv)
Condensation
Q-6 The substance that sublimates is:
(i)
Wax
(ii)
Ice
(iii)
Pure iron
(iv)
Iodine
Q-7 The state in which the intermolecular force is the least is:
(i)
Liquid
(ii)
Solid
(iii)
Gas
(iv)
Plasma
Q-8 When ice melts heat is _________.
(i)
Given out
(ii)
Taken in
(iii)
Unchanged
(iv)
None of these
Q-9 Gases have:
(i)
Fixed shape and volume
(ii)
Variable shape and volume
(iii)
Variable shape but fixed volume
(iv)
Fixed shape but variable volume
Q-10 The three states of matter depends on:
(i)
Temperature
(ii)
Force
(iii)
Potential energy
(iv)
Volume
Q-11 When water is cooled, the motion of its particles become:
(i)
Slow
(ii)
Fast
(iii)
Unchanged
(iv)
None of these
Q-12 The continuous motion of particles in solids, liquids and gases is observed as:
(i)
Potential model of atoms
(ii)
Kinetic model of matter
(iii)
Kinetic model of atoms
(iv)
Potential model of matter
Q-13 Intermolecular forces can be defined as force between:
(i)
Two solids
(ii)
Two substances
(iii)
Two atoms or molecules
(iv)
Liquids and gases
Q-14 Matter consists of tiny particles called:
(i)
Molecules
(ii)
Atoms
(iii)
Ions
(iv)
Elements
Q-15 Which of the following can be described as being 'fluid'?
(i)
Solids
(ii)
Liquids
(iii)
Gases
(iv)
Both b and c
Chapter-2 Physical and Chemical Changes
Q-1
Classify the following as physical or chemical change.
(i) Heating iron and sulphur.
(ii) Making cheese from milk.
(iii) Making wine from grapes.
(iv) Obtaining milk powder from milk.
(v) Burning of paper.
(vi) Making cloth from yarn.
(vii) Making paper from pulp.
(viii) Shredding a sheet of paper.
(ix) Printing on a sheet of paper.
(x) Digestion of food.
Q-2
State whether the following statements are true or false:
(i) Land and Sea breeze are a non-periodic change.
(ii) Phases of the Moon is a periodic change.
(iii) Making wine from grapes.
(iv) Obtaining milk powder from milk.
Multiple Choice Questions
Q-1 Which of the following is a characteristic of a physical change?
(i)
It can be reversed easily
(ii)
It is a permanent change
(iii)
New substances are formed
(iv)
None of these
Q-2 Growth of a crystal is an example of:
(i)
Change of state
(ii)
Irreversible change
(iii)
Physical change
(iv)
Chemical change
Q-3 Changes that affect properties like shape, size, state and colour are called:
(i)
Physical change
(ii)
Periodic change
(iii)
Natural change
(iv)
All of these
Q-4 When salt dissolves in water, it loses its:
(i)
Salty taste
(ii)
Crystalline shape
(iii)
Neither a nor b
(iv)
All of these
Q-5 Which of the following is a physical change?
(i)
Change of state
(ii)
Photosynthesis
(iii)
Respiration
(iv)
All of these
Q-6 When sulphur is heated with iron the change is chemical because:
(i)
A new substance is formed
(ii)
Heat and light is given out
(iii)
The change is permanent
(iv)
All of these
Q-7 The burning of magnesium is:
(i)
A fast change
(ii)
Chemical change
(iii)
Exothermic
(iv)
All of these
Q-8 The daily rise and fall of tides is a:
(i)
Periodic change
(ii)
Non-periodic change
(iii)
Natural change
(iv)
Both a and c are true
Q-9 Action of heat on copper carbonate is a chemical change because:
(i)
A gas carbon dioxide is evolved
(ii)
A new black substance is formed
(iii)
The change is permanent
(iv)
All of these
Q-10 When a magnesium ribbon burns in oxygen it gives out:
(i)
Heat
(ii)
Light
(iii)
Sound
(iv)
Only a and b
Q-11 Water can be decomposed by passing _____ through it.
(i)
Light
(ii)
Electricity
(iii)
Neither
(iv)
Both
Q-12 An example of a photochemical reaction is:
(i)
Respiration
(ii)
Electrolysis
(iii)
Photosynthesis
(iv)
All of these
Q-13 Energy changes in a chemical reaction may be:
(i)
Heat
(ii)
Sound
(iii)
Electricity
(iv)
All of these
Q-14 A chemical reaction may involve the formation of:
(i)
Gases
(ii)
Precipitates
(iii)
Sound
(iv)
All of these
Q-15 When water freezes into ice, the density of ice is ________ that of water:
(i)
Less than
(ii)
More than
(iii)
Same as
(iv)
None of these
Chapter-3 Elements, Compounds and Mixtures
Q-1
Classify the following under element, compound and mixture.
(i) Soil
(ii) Uranium
(iii) Starch
(iv) Silver coin
(v) Amalgamated zinc
(vi) Mercury vapour
(vii) Blood
(viii) Bricks
(ix) Gold
(x) Seawater
Q-2
Define the following terms and answer the associated questions.
(i) Element: Name the four main categories into which elements can be divided. Also give two examples of each.
(ii) Compound: Give three reasons to prove that water is a compound .
(iii) Mixture: Give two examples of a homogeneous mixture and two examples of a heterogeneous mixture.
(iv) Filtration: Give an example of a mixture in which the components can be separated by this technique.
(v) Fractional distillation: Can a mixture of chloroform and water be separated by this method? Explain.
Q-3
Give one example each of:
(i) Solid-solid homogeneous mixture.
(ii) Solid-solid heterogeneous mixture.
(iii) Solid-liquid heterogeneous mixture.
(iv) Solid-liquid homogeneous mixture.
(v) Liquid-liquid homogeneous mixture.
(vi) Liquid-liquid heterogeneous mixture.
Q-4
Complete the following table based on separation techniques. The first one is done for you.
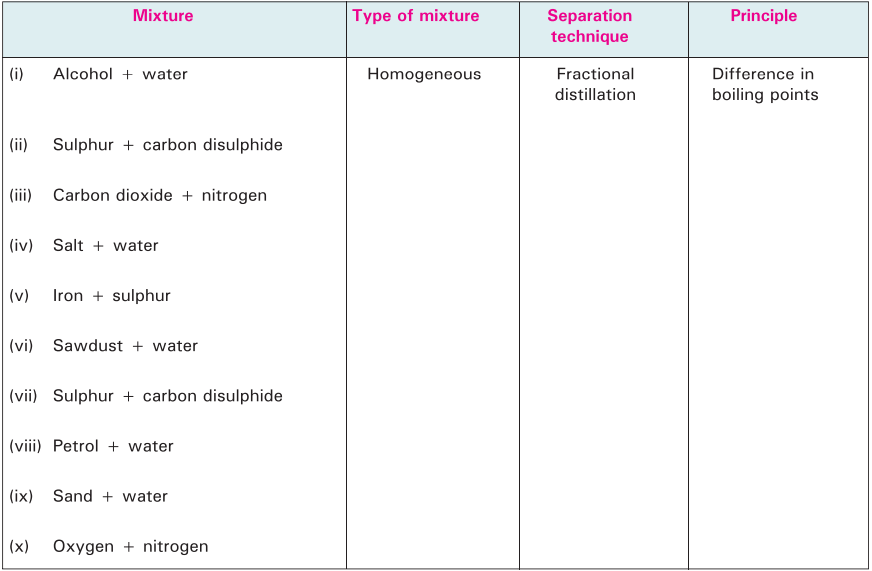
Multiple Choice Questions
Q-1 The following elements show polyatomicity:
(i)
Sulphur
(ii)
Boron
(iii)
Phosphorus
(iv)
All of these
Q-2 There are ________elements known to us.
(i)
125
(ii)
91
(iii)
118
(iv)
101
Q-3 The symbol of an element can be:
(i)
First letter of its English name
(ii)
First two letters of its English name
(iii)
Derived from its Latin name
(iv)
All of these
Q-4 Which of the following element is triatomic?
(i)
Phosphorus
(ii)
Argon
(iii)
Ozone
(iv)
Chlorine
Q-5 Which of the following statement is true?
(i)
Mixtures are always homogeneous
(ii)
Water is a mixture
(iii)
Air is a compound
(iv)
Non-metals are elements
Q-6 Which statement is NOT true for compounds?
(i)
They are never homogeneous
(ii)
Fixed composition by weight
(iii)
Can be shown by a formula
(iv)
Its formation involves energy change
Q-7 When iron and sulphur are mixed and heated together, the product formed is a:
(i)
Mixture
(ii)
Compound
(iii)
Element
(iv)
None of these
Q-8 The water molecule is made up____ of atoms:
(i)
2
(ii)
3
(iii)
1
(iv)
4
Q-9 The formula for common salt is:
(i)
NaCl 2
(ii)
NaCl
(iii)
CINa
(iv)
Na2 Cl
Q-10 Which of the following is a liquid metal?
(i)
Gallium
(ii)
Mercury
(iii)
Francium
(iv)
All of these
Q-11 There are _________ gaseous elements known to us.
(i)
9
(ii)
10
(iii)
11
(iv)
12
Q-12 There are ____________ liquid elements known to us.
(i)
6
(ii)
3
(iii)
4
(iv)
2
Q-13 Which of the following is NOT a homogeneous mixture?
(i)
Alcohol + water
(ii)
Gun powder
(iii)
Ammonia + hydrogen
(iv)
Sulphur + carbon disulphide
Q-14 Which of the following is a homogeneous mixture?
(i)
Petrol + kerosene
(ii)
Petrol + water
(iii)
Oil + water
(iv)
Sulphur trioxide + water
Q-15 Chromatography can be used to separate pigments of:
(i)
Ink
(ii)
Flower
(iii)
Chlorophyll
(iv)
All of these
Chapter-4 Atomic Structure
Q-1
Answer the following Questions.
(i) What does the word 'atom' mean?
(ii) According to Dalton's Atomic Theory, the atom is indivisible. Do later findings support this theory?
(iii) State three properties of isotopes.
(iv) Name the isotope of hydrogen that has two neutrons.
(v) Explain why the relative atomic mass of chlorine is a fraction.
(vi) What are atoms with complete octet or duplet called?
Q-2
Explain the following terms:
(i) Atomic Number
(ii) Mass Number
(iii) Electronic Configuration
(iv) Isotope
(v) Valency
(vi) Valence Electrons
Q-3
Which of the following atoms have a complete octet?
(i) Argon
(ii) Calcium
(iii) Neon
(iv) Potassium
(v) Krypton
(vi) Helium
Q-4
XN is the metal nitride. Write:
(i) the ion of X
(ii) number of valence electrons in the atom of X.
(iii) its formula with phosphate, chromate, carbide
Q-5
Fill in the blanks:
(i) A _________ bond is formed when atoms mutually share electrons.
(ii) An isotope of hydrogen with no neutrons is ___________.
(iii) The plum pudding model of the atom was proposed by ________.
(iv) A chloride ion has _________ electrons.
(v) Isotopes of the same element have the same _________ number.
Multiple Choice Questions
Q-1 Which of the following is a noble gas?
(i)
2, 8, 1
(ii)
2
(iii)
2, 8, 8
(iv)
Both b and c
Q-2 Which of the following will not combine to form a compound?
(i)
Radon
(ii)
Carbon
(iii)
Nitrogen
(iv)
Magnesium
Q-3 Which statement is true for an atom with 3 valence electrons?
(i)
It is a metal
(ii)
It is a non-metal
(iii)
It is a noble gas
(iv)
It is a metalloid
Q-4 Which statement is true for an element with atomic number 7?
(i)
It is a non-metal
(ii)
It is a metalloid
(iii)
It is a noble gas
(iv)
It is a metal
Q-5 The ions of ________ have the same electronic configuration.
(i)
Sodium and potassium
(ii)
Potassium and chlorine
(iii)
Sulphur and sodium
(iv)
Fluorine and chlorine
Q-6 An atom having atomic number 13 will _____ 3 electrons to form a ______
(i)
Lose, anion
(ii)
Lose, anion
(iii)
Gain, anion
(iv)
Gain, cation
Q-7 An atom having atomic number 18 will _____ electrons.
(i)
Lose electrons
(ii)
Gain electrons
(iii)
Neither lose nor gain
(iv)
None of these
Chapter-5 Language of Chemistry
Q-1
Write the following equations and balance them.
(i) Copper + nitric acid ➔ Copper(II) nitrate + water + nitric oxide
(ii) Aluminium + sodium hydroxide + water ➔ Sodium aluminate + hydrogen
(iii) Iron + sulphuric acid ➔ Ferrous sulphate + hydrogen
(iv) Zinc sulphide + oxygen ➔ Zinc oxide + sulphur dioxide
(v) Magnesium nitride + water➔ Magnesium hydroxide + ammonia
Q-2
Write the formula and valency of the following radicals:
(i) Ferric
(ii) Ferricyanide
(iii) Carbide
(iv) Sulphide
(v) Oxide
(vi) Phosphate
Q-3
Write the formulae of the following compounds:
(i) Potassium chloride
(ii) Calcium chloride
(iii) Sodium sulphide
(iv) Potassium permanganate
(v) Chromium sulphate
(vi) Nickel nitrate
(vii) Copper(II) carbonate
(viii) Sodium nitrate
(ix) Calcium bicarbonate
(x) Aluminium sulphate
Chapter-6 Chemical Reactions
Q-1
Write the name and formula of the compound formed when the following react:
(i) Iron and sulphur
(ii) Hydrogen and oxygen
(iii) Magnesium and oxygen
(iv) Sodium and chlorine
(v) Hydrogen and sulphur
(vi) Lead and bromine
Q-2
State the type of chemical reaction that takes place in the following situations:
(i) Metals lose their lustre on exposure to air.
(ii) Formation of acid rain.
(iii) Relieving stomach acidity with soda bicarbonate.
(iv) A coal fire burning.
(v) Electrolysis of water.
(vi) Copper carbonate is heated in a test-tube.
(vii) Silver articles turn black.
(viii) Holes appear on the iron pot in which copper sulphate is stored.
(ix) Farmers add calcium hydroxide to the soil.
(x) Iron articles get corroded.
Q-3
State how you could separate:
(i) Silver chloride from a mixture of silver chloride and lead(II) chloride.
(ii) Copper(II) chloride from a mixture of copper(II) chloride and copper(II) hydroxide.
Q-4
Answer these Questions.
(i) Define an oxide.
(ii) Identify the oxide and state whether it is an acidic, basic, amphoteric or neutral oxide.
(a) The oxide is yellow when hot and white when cold.
(b) It is a reddish-brown gas.
(c) It is black, and is formed by the action of heat on copper nitrate.
(d) It is decomposed on heating to form a metal and oxygen.
(e) It is formed by the synthesis of two neutral gases present in the air
(iii) Give a balanced equation to prepare each oxide named in (ii).
(iv) State one property of each of the four types of oxide.
Q-5
The following substances are burnt in air (oxygen). Give balanced equations for each reaction taking place.
If the product formed in each of the reaction is added to water, what would be the effect of the solution (if any) formed on litmus?
(i) Carbon
(ii) Calcium
(iii) Sulphur
(iv) Potassium
(v) Phosphorus
(vi) Magnesium
Q-6
Give a balanced equation for the reaction of zinc oxide with:
State the property of zinc oxide illustrated by these reactions .
(i) Dilute hydrochloric acid
(ii) Hot concentrated caustic potash
Q-7
Answer these questions.
(i) Explain the term 'acid anhydride'.
(ii) Name a mixed acid anhydride.
(iii) Name the anhydride of sulphuric acid.
Multiple Choice Questions
Q-1 When ammonium chloride is added to water in a beaker, the beaker becomes:
(i)
Remains same
(ii)
None of these
(iii)
Warm
(iv)
Cold
Q-2 Which statement is true for the action of heat on mercuric oxide?
(i)
It is a decomposition reaction
(ii)
Products are mercury and oxygen
(iii)
A silver mirror appears in the test-tube
(iv)
All these are true
Q-3 When sodium hydroxide is added to ferrous chloride, the colour of precipitate formed is:
(i)
White
(ii)
Blue
(iii)
Dirty green
(iv)
Reddish-brown
Q-4 Which of the following is a natural indicator?
(i)
Litmus
(ii)
Beetroot juice
(iii)
China-rose
(iv)
All of these
Q-5 Which is a synthetic indicator?
(i)
Methyl orange
(ii)
Red cabbage
(iii)
China-rose
(iv)
Litmus
Q-6 Phenolphthalein turns pink in the presence of:
(i)
Acid
(ii)
Alkali
(iii)
Salt
(iv)
None of these
Q-7 The acid present in the stomach is:
(i)
Hydrochloric acid
(ii)
Sulphuric acid
(iii)
Nitric acid
(iv)
Sulphurous acid
Q-8 The acid used in fire extinguishers is:
(i)
Hydrochloric acid
(ii)
Sulphuric acid
(iii)
Nitric acid
(iv)
Sulphurous acid
Chapter-7 Hydrogen
Q-1
Give balanced equations to prepare hydrogen starting with each of the following substances:
(i) A dilute acid
(ii) Cold water
(iii) Acidulated water
(iv) A hot concentrated alkali
(v) Steam
(vi) A metal hydride
Q-2
You are provided with dilute hydrochloric acid and samples of the following metals:
Sodium, calcium, magnesium, iron, zinc, lead, copper, silver
Name:
(i) The metals that would not liberate hydrogen from the acid.
(ii) The metal(s) that would react explosively with the acid.
(iii) A metal that is above hydrogen in the activity series, yet does not displace hydrogen. Also give a reason for this behaviour.
(iv) A metal that liberates hydrogen very slowly from the acid.
(v) A metal that reacts moderately with the acid.
Q-3
Answer these questions.
(i) Name the scientist who proved that hydrogen is a compound and not an element.
(ii) If instead of dilute hydrochloric acid, you are provided with nitric acid, would hydrogen be liberated? Explain.
(iii) Name two large scale methods of obtaining hydrogen.
(iv) What is water gas?
(v) How is water gas obtained?
(vi) Name the products formed when steam is reacted with water gas at 1000°C in the presence of a catalyst. Also give a balanced equation for the reaction.
(vii) Name the catalyst used in 6 (vi).
(viii) How are the products obtained in 5 (vi) separated?
(ix) State the conditions under which hydrogen reacts with the following:
(i) Chlorine (ii) Nitrogen (iii) Sulphur (iv) Copper(II) oxide
Give balanced equations for the reactions taking place in (ix).
Q-4
In the laboratory preparation of hydrogen,
(i) How is the gas collected? Explain why this method is used.
(ii) How is the gas dried?
(iii) What precaution must be taken to ensure safe preparation and collection of the gas?
(iv) How would you confirm that the gas collected is hydrogen?
Q-5
State one property of hydrogen:
(i) That made it in useful in meteorological balloons.
(ii) Due to which it is no longer used in meteorological balloons.
(iii) Due to which it is used in metallurgy.
(iv) That makes it a good fuel.
Q-6
State what you would observe when:
(i) Hydrogen is passed over heated copper(II) oxide.
(ii) Hydrogen and chlorine react in direct sunlight.
Q-7
Fill in the blanks:
(i) Hydrogen, along with nitrogen is used in the manufacture of ________ gas.
(ii) The process by which vegetable oils are converted into fat is called ________ .
(iii) Hydrogen ______ (does/does not) support combustion.
(iv) The ______ flame is used for cutting and welding of metals.
(v) Hydrogen burns with a pale ______ flame.
Multiple Choice Questions
Q-1 Hydrogen burns with a______ flame.
(i)
Yellow
(ii)
Blue
(iii)
Orange
(iv)
None of these
Q-2 Which of these is the large scale method of preparing hydrogen?
(i)
Haber Process
(ii)
Bosch Process
(iii)
Electrolysis
(iv)
Both band c
Q-3 Hydrogen is liberated when dilute HCI is added to:
(i)
Silver
(ii)
Copper
(iii)
Sodium
(iv)
Lead
Q-4 Which statement is true about hydrogen?
(i)
Combustible, supporter of combustion
(ii)
Non-combustible, non-supporter of combustion
(iii)
Non-combustible, supporter of combustion
(iv)
Combustible, non-supporter of combustion
Q-5 The reaction between hydrogen and chlorine proceeds smoothly in:
(i)
Direct sunlight
(ii)
Diffused sunlight
(iii)
Darkness
(iv)
All of these
Q-6 Hydrogen turns moist:
(i)
Red litmus blue
(ii)
Blue litmus red
(iii)
Red litmus red
(iv)
None of these
Q-7 When hydrogen reacts with nitrogen the catalyst used is:
(i)
Nickel
(ii)
Platinum
(iii)
Powdered iron
(iv)
lron(III) oxide
Q-8 The gases used in welding torch are:
(i)
Hydrogen and nitrogen
(ii)
Hydrogen and oxygen
(iii)
Hydrogen and carbon dioxide
(iv)
Oxygen and nitrogen
Q-9 Water gas is a mixture of:
(i)
Carbon monoxide and nitrogen
(ii)
Carbon monoxide and hydrogen
(iii)
Carbon dioxide and hydrogen
(iv)
Carbon and steam
Q-10 Which of the following will yield hydrogen with zinc?
(i)
Sodium hydroxide
(ii)
Steam
(iii)
Hydrochloric acid
(iv)
All of these
Q-11 Sodium metal is not used in the laboratory preparation of hydrogen because it is very:
(i)
Expensive
(ii)
Reactive
(iii)
Non-reactive
(iv)
None of these
Q-12 Hydrogen is used in metallurgy because it is:
(i)
Oxidising agent
(ii)
Reducing agent
(iii)
Combustible
(iv)
Inflammable
Q-13 Compounds of hydrogen with metal is called:
(i)
None of these
(ii)
Hydroxide
(iii)
Hydrocarbon
(iv)
Hydride
Q-14 This metal reacts reversibly with steam to yield hydrogen:
(i)
Aluminium
(ii)
Magnesium
(iii)
Iron
(iv)
Zinc
Q-15 Hydrogen is no longer used in meteorological balloons because it is:
(i)
Heavy
(ii)
Inflammable
(iii)
Light
(iv)
Insoluble
Chapter-8 Water
Q-1
Define the following:
(i) Solute
(ii) Solvent
(iii) Solution
(iv) Solubility
Q-2
Fill in the blanks:
(i) When a solute completely dissolves in a solvent at a particular temperature, the mixture of a solute and a solvent is called a ____________ solution.
(ii) If more of the solute can dissolve in the solvent at a particular temperature, then the solution is said to be an ____________ solution.
(iii) If no more of the solute can dissolve in the solvent at a particular temperature, then the solution is said to be a ____________ solution.
(iv) The weight in grams of the solute that will saturate 100 g of the solvent at that particular temperature is said to be the ____________ of the solute.
(v) An increase in temperature results in ____________ in the solubility of the solute in the solvent.
Q-3
Identify the following as a true solution, colloidal solution or a suspension:
(i) It is a cloudy, homogeneous mixture.
(ii) It is a transparent, homogeneous mixture.
(iii) It is a cloudy, heterogeneous mixture.
(iv) Size of solute particle is greater than 10-5 cm.
(v) Solute particles can be seen by a powerful microscope.
(vi) Solute particles cannot be seen by the naked eye.
(vii) Solute particles can be seen with the naked eye.
(viii) Size of solute particle is less than 10-9 cm.
(ix) Size of solute particle is between 10-9 cm and 10-5 cm
(x) Blood and milk are examples of this type of solution.
Q-4
What is water of crystallisation? Give the name and formula of a compound:
(i) That contains water of crystallisation.
(ii) That does not contain water of crystallisation.
Q-5
Answer these Questions.
(i) State three factors that influence the rate of solubility of a solute in a solvent.
(ii) State two differences between hard water and soft water.
(iii) Name two substances that cause:
(a) Temporary hardness of water
(b) Permanent hardness of water
(iv) State two: (a) Advantages of hard water (b) Drawbacks of hard water
(v) What is meant by softening of water?
(vi) State a method by which temporary hard water can be softened. Give a balanced equation to show the reaction taking place.
(vii) State a method by which permanent hard water can be softened. Give a balanced equation to show the reaction.
Q-6
Explain the following observations:
(i) White copper sulphate turns blue when water is added to it.
(ii) Sodium reacts vigorously with water and the solution formed turns red litmus blue.
(iii) Colloidal and suspension solutions scatter light.
(iv) Copper does not react with water or steam.
(v) Soap fails to lather in hard water.
Multiple Choice Questions
Q-1 Which of these metals will not react with steam?
(i)
Sodium
(ii)
Iron
(iii)
Magnesium
(iv)
Copper
Q-2 Which of these is a method of softening hard water?
(i)
Boiling
(ii)
Freezing
(iii)
Evaporation
(iv)
Both a and c
Q-3 Alkali is formed when the following metals are added to cold water:
(i)
Na, Al, K
(ii)
Na, Ca, K
(iii)
Na, Mg, K
(iv)
Na, K, Zn
Q-4 The correct order of metals in decreasing reactivity is:
(i)
K >Na> Ca >Mg> Al >Zn> Fe> Pb > Cu> Hg > Ag > Au
(ii)
K >Na> Ca >Al> Mg >Zn> Fe> Pb > Cu> Hg > Ag > Au
(iii)
K >Na> Ca > Mg> Al >Zn> Fe> Pb > Cu> Ag > Hg > Au
(iv)
K >Na> Ca > Mg> Al > Fe > Zn > Pb > Cu> Hg > Ag > Au
Q-5 This metal reacts reversibly with steam:
(i)
Al
(ii)
Zn
(iii)
Fe
(iv)
Mg
Q-6 These are colloidal solutions:
(i)
Milk, glue, ink
(ii)
Salt solution, aq. copper(II) sulphate
(iii)
Smoke in air, sawdust in water
(iv)
None of these
Q-7 These are suspension solutions:
(i)
Muddy water
(ii)
Smoke particles in air
(iii)
Oil dispersed in water
(iv)
All of these
Chapter-9 Carbon and its Compounds
Q-1
Answer these Questions.
(i) Write the symbols of the three isotopes of carbon.
(ii) Which isotope of carbon is used as a standard for comparison of atomic weights?
(iii) Which isotope of carbon is used for dating of prehistoric objects?
(iv) Define allotropy and state three reasons why it occurs.
(v) Define destructive distillation.
(vi) Explain how destructive distillation enhances the chemical activity of a substance, for example, wood.
(vii) Name the naturally occurring form of amorphous carbon and briefly explain how it was formed.
(viii) Name one allotrope of carbon that is a good conductor of electricity.
Q-2
Name the allotropic form of carbon that is used:
(i) As a fuel
(ii) In metallurgy
(iii) In water filters
(iv) In nuclear reactors
(v) As an abrasive
(vi) As rods for electric arcs
(vii) As a gem
(viii) As a dry lubricant for heated machine parts
Q-3
Explain briefly:
(i) When carbon dioxide is heated with coke, the coke eventually disappears.
(ii) Lead monoxide, when heated in a charcoal cavity, leaves a shining globule.
(iii) Graphite is used in the manufacture of refractory crucibles.
(iv) Graphite conducts electricity while diamond does not, although both are the forms of carbon.
Q-4
State whether the following statements are true or false:
(i) If diamond is heated above 1500°C in the absence of air, it is transformed into graphite.
(ii) Diamond is opaque to X-rays.
Q-5
State one use each of:
(i) Black diamonds
(ii) Diamond dust
(iii) Coke
(iv) Graphite
Q-6
Fill in the blanks:
(i) ________ is the most stable allotrope of carbon.
(ii) _______ acid oxidises graphite to carbon.
(iii) ________ is the hardest known substance.
(iv) When heated above _______ °C diamond is transformed into graphite.
(v) The layers of graphite are held by weak __________forces.
Q-7
Answer these Quesions
(i) Give balanced equation to prepare carbon dioxide in the laboratory. Name the reactants used.
(ii) What is meant by photosynthesis?
(iii) Explain the significance of photosynthesis.
(iv) Explain the Greenhouse effect.
Q-8
State what you would observe when:
(i) Dilute hydrochloric acid is added to marble chips.
(ii) Carbon dioxide is bubbled through limewater.
(iii) Moist blue litmus is introduced into a jar containing carbon dioxide.
(iv) A burning splinter is introduced into a jar containing carbon dioxide.
(v) Limewater is left exposed to air for a long time.
Q-9
Explain why:
(i) Carbon dioxide is collected by the upward displacement of air.
(ii) Carbon dioxide is used in fire extinguishers.
(iii) Carbon dioxide is used in aerated drinks.
Q-10
Give balanced equations to obtain carbon monoxide by the following methods:
(i) Reduction of a metal oxide.
(ii) By oxidation of a non-metal.
(iii) Reduction of a non-metallic oxide.
Q-11
Explain briefly:
(i) It is dangerous to start a car engine in a closed garage
(ii) A blue flame is often observed on top of a coal fire.
(iii) It is dangerous to sleep in an ill ventilated room with a coal fire burning.
(iv) Carbon monoxide is used in metallurgy.
(v) Carbon monoxide forms addition compounds.
Multiple Choice Questions
Q-1 Which of these will liberate CO2 when HCI is added to it?
(i)
Sodium sulphate
(ii)
Sodium chloride
(iii)
Sodium carbonate
(iv)
Sodium
Q-2 Which of these is a crystalline form of carbon?
(i)
Graphite
(ii)
Coke
(iii)
Charcoal
(iv)
Soot
Q-3 Which of these is an amorphous form of carbon?
(i)
Soot
(ii)
Coal
(iii)
Coke
(iv)
All of these
Q-4 Which statement is true for diamond?
(i)
It is the hardest known substance
(ii)
It has a high refractive index
(iii)
There is no known solvent for diamond
(iv)
All are true statements
Q-5 Lime water turns milky due to the formation of insoluble:
(i)
Calcium sulphate
(ii)
Calcium carbonate
(iii)
Calcium bicarbonate
(iv)
None of these
Q-6 Which of the following is NOT a reducing agent?
(i)
Charcoal
(ii)
Hydrogen
(iii)
Carbon monoxide
(iv)
Carbon dioxide
Q-7 Which of the following is a product of destructive distillation of coal?
(i)
Coal gas
(ii)
Coal tar
(iii)
Ammoniacal liquor
(iv)
All of these
Q-8 A mixture of carbon monoxide and nitrogen is called:
(i)
Water gas
(ii)
Producer gas
(iii)
Coal gas
(iv)
Natural gas
Q-9 Which of the following is a green house gas?
(i)
Methane
(ii)
Carbon dioxide
(iii)
Water vapour
(iv)
All of these
Q-10 Carbon monoxide forms a stable compound with haemoglobin of the blood called:
(i)
Oxyhaemoglobin
(ii)
Carboxyhaemoglobin
(iii)
Carbaminohaemoglobin
(iv)
None of these
Q-11 Carbon dioxide is:
(i)
Combustible, non-supporter of combustion
(ii)
Non-combustible, non-supporter of combustion
(iii)
Non-combustible, supporter of combustion
(iv)
Combustible, supporter of combustion
Q-12 Lime water is a saturated solution of:
(i)
Lime juice
(ii)
Calcium hydroxide
(iii)
Quick lime
(iv)
Calcium sulphate
Q-13 Carbon dioxide is an acid because it reacts with:
(i)
Alkalis to form salt and water
(ii)
Dissolves in water to form an acid
(iii)
Oxidises coke to carbon monoxide
(iv)
Both a and b